
FDA Fast-Track Covid-19 Vaccine To Save Trump’s Presidency?

FDA
Fast-Track Covid-19 Vaccine To Save Trump’s Presidency?
The head of the US
Food and Drug Administration (FDA), Stephen Hahn is willing to bypass the
standard drug approval process to authorize a Covid-19 vaccine. Since his
statement to the Financial Times on Sunday, rumors had been spreading that Dr
Hahn made that decision to help with US President Donald Trump with his
November Presidential election.
Dr Hahn said that
the administration is prepared to authorize an effective vaccine even before
Phase Three trials were completed, under the circumstances that the benefits
are believed to outweigh the risks. According to him, it is up to the vaccine
developer to apply for approval, before the FDA determines whether the
application is appropriate. Dr Hahn’s announcement to decide on the approval is
one of the most important and sensitive in US public health history, given the
severeness of the Covid-19.
Previously, China
and Russia had approved vaccines without waiting for the completion of Phase
Three trials. Their decision had faced strong criticism by public health
officials, including the US, as the Phase Three trials are the largest and most
rigorous tests for a new drug. However, Dr Hahn insisted otherwise, saying that
it is possible to issue and emergency authorization for the use of the drug by
certain groups first, rather than an overall blanket approval. Dr Hahn claimed
that the emergency use is unlike the full approval as the legal, medical, and
scientific standard for it is more beneficial during a public health emergency.
The sudden change
in the FDA’s stand gained controversy among the public, leading to the rumors
that they are doing it in favour of the President. This comes after the reports
that President Trump wants an effective vaccine before November’s election to
boost his political popularity. From his defense, Dr Hahn mentioned that it is
coincident that the pandemic and the political season are converging, but the
FDA will only act out of science, medicine, and data decision.
Last Saturday,
President Trump accused the FDA of their slow progress in approving new
treatment methods for the Covid-19 in an effort to damage his political
position. The next day, Dr Hahn and President Trump jointly announced the
emergency authorization for convalescent plasma, which uses the plasma from
recovered Covid-19 patients as a treatment. Dr Hahn also mentioned that the
plasma treatment could save the lives of 35 out of 100 patients, but data
suggest only 5 out of 100. Shortly after, Dr Hahn was criticized for
overstating the benefits.
Follow Regain capital
latest articles
-
- Mar 11,2022
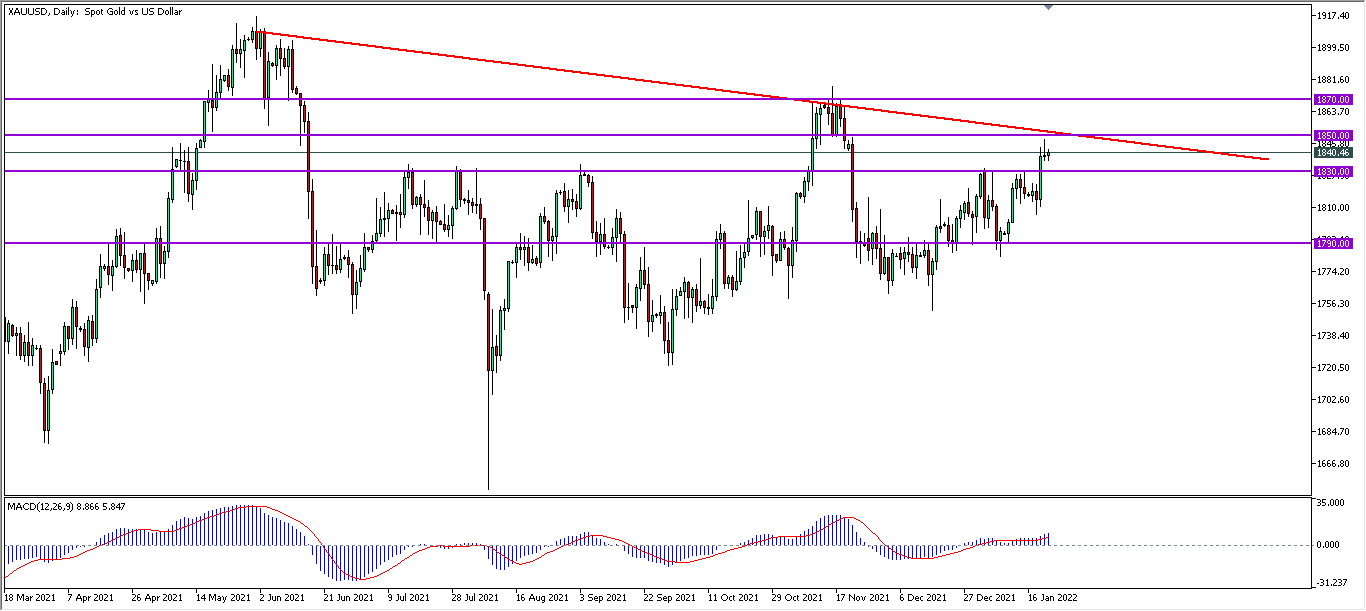
-

-

-
- Sep 09,2021

-
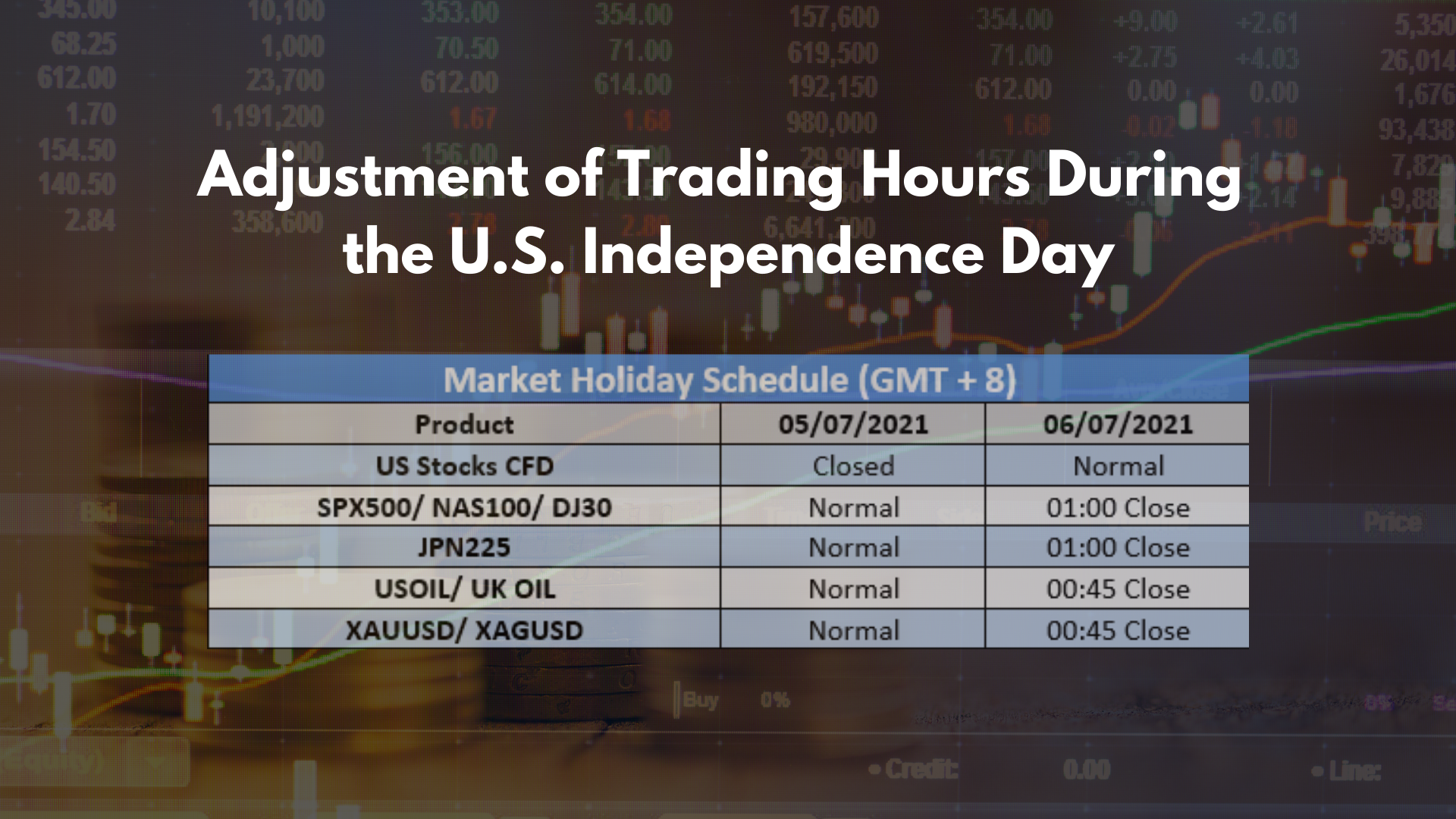
-
- Oct 22,2020
































